In arson cases, accelerants such as ignitable liquids are often used to initiate or accelerate the fire. Hence, the detection of ignitable liquid residues (ILRs) is one of the important steps in fire investigation in order to consider the possibility of arson. Fire debris samples collected from a fire scene will be analysed based on the ASTM Standard Test Methods to identify if there is any ILRs present. Prior to analysis, the ignitable liquid residue must first be in a vapor or volatile liquid form. There are several different sample preparation techniques such as Passive Headspace Concentration with Activated Charcoal, Tenax and Solid Phase Microextraction (SPME). The most commonly used technique to analyse ILRs is by using Gas Chromatography – Mass Spectroscopy (GC-MS). Gas chromatography allows the separation, identification and determination of volatile hydrocarbon mixtures based on the differences of boiling point. Briefly, a GC is functioned by introducing a sample into the inlet via the injection port. Carrier gas flows through the inlet into the column taking the sample along with it. The column is enclosed in a temperature-controlled oven. The sample is separated as it travels through the column. The separated components then exit the column and enter the detector, where an electronic signal is generated.
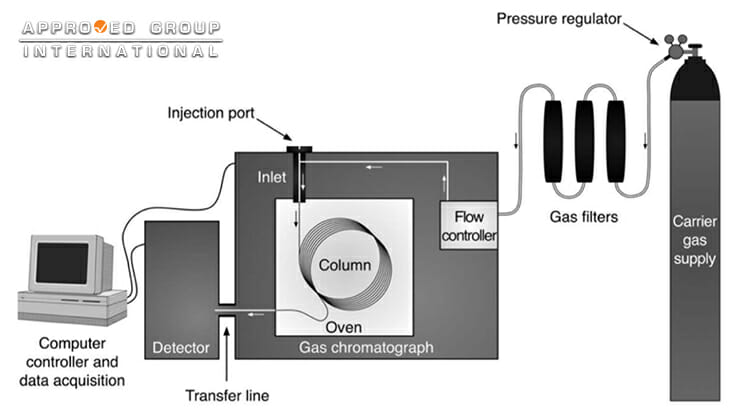
The separation involves dissolving the sample in a mobile phase (carrier gas) and the mobile phase is then forced through an immiscible stationary phase (column). Components that are strongly held by the stationary phase will move slowly with the flow of mobile phase while components that are held weakly will travel rapidly. As a result, the sample components separate. The data obtained are presented as a chromatogram, a plot of retention time against intensity which contains peaks corresponding to the separated compounds from the fire debris sample. The most commonly used detector for gas chromatography is mass spectrometry (MS); an analytical technique that breaks apart each compound into small sub-molecular pieces and establish the chemical structure of the organic molecule by counting those pieces. These ions are then sorted according to their mass-to-charge ratio (m/z), detected, organized and presented in the form of a mass spectrum. MS is able to provide structural information that is sufficient for the identification of separated hydrocarbon component in fire debris analysis. Separation can be carried out in the GC while MS could provide structural information regarding the separated compounds. The combination of GC and MS allows it to display mass spectrum for each peak and for specific ions. This means that it can display mass spectrum specifically for the selected ion, ignoring other peaks. For example, Figure 2 below shows a typical chromatogram of a kerosene using MS as the detector. The chromatogram shows all the major peaks, showing that this kerosene has an alkane range from heptane (C7) to hexadecane (C16). Kerosene contains different types of hydrocarbons: alkanes, cycloalkanes and aromatics.
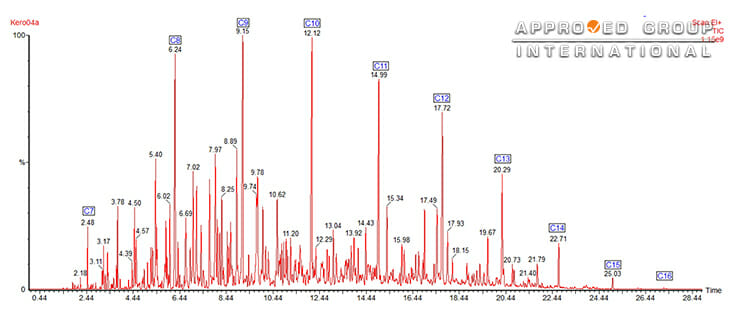
By using selective ion monitoring, a scanning mode of MS, these different classes of compounds can be separated. Only a limited mass-to-charge ratio range is detected by the instrument, instead of the full spectrum range.
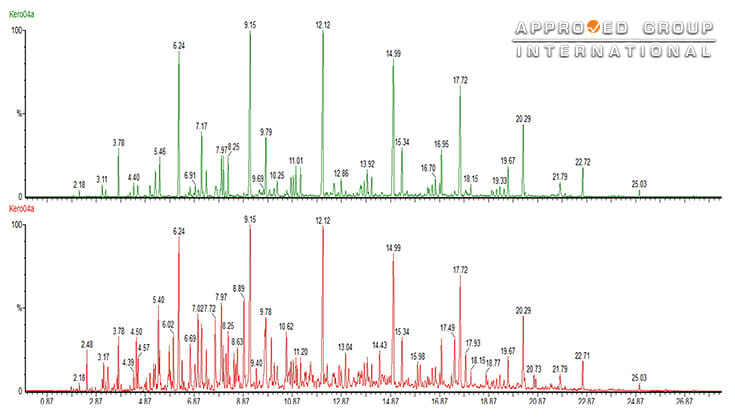
The red chromatogram in Figure 3 shows the whole range of compounds in the kerosene. By using selective ion monitoring, profile of the straight chain and branched chain alkanes can be separated and showed in a different chromatogram, as shown in the green chromatogram. The combination of GC and MS has become the favourable technique for fire debris analysis due to its ability to yield molecular information. Other than that, GCMS also contains a data library of compounds, making it possible to search for particular fragments, groups of fragments of molecular ions, and is particularly well suited to identify aliphatic and aromatic hydrocarbon peaks and mixtures.
Arsonists can run but cannot hide their modus operandi from the keen fire investigation skills of AGI’s forensic consultants.























